Philosophers and scientists alike have long been intrigued by the concept of an atom. Its name derives from Greek words meaning indefinable or invincible (atomos), recalling ancient ideas about unbreakable units of matter which made up our universe. But today our understanding has evolved significantly and are considered basic elements. In this 2000 word piece we explore their development within theoretical atomic physics theory as well as structure of atoms as part of shaping today’s physical universe.
History of Atomic Theory
Ancient Philosophical Notions
Humankind first attempted to understand what an atom was during prehistoric times when philosophers from Greece and India attempted to understand what makes up matter’s fundamental makeup. For instance, Democritus from Greece as well as Kanada from India both believed matter was composed of indestructible particles known as anatomos. Democritus first used “atomos” as an adjective referring to these non-cuttable particles he called anatomias (meaning: an unbreakable particle).
Alchemy and Mattertransformation
From medieval Europe through to Renaissance Italy, alchemy was an influential form of chemical philosophy that formed part of its development. Alchemists found ways to convert base metals like lead into nobler metals like gold. Additionally they discovered various chemical processes and chemicals during this process and left behind symbolic yet mysterious quests which laid the groundwork for modern chemistry by investigating properties and effects of matter.
Dalton’s Atomic Theory
Scientific understanding of atoms formally began evolving at the start of the 19th century when John Dalton, an English physicist and chemist from Oxfordshire, advanced Dalton’s Atomic Theory in 1803. According to Dalton’s theory:
All matter is composed of microscopic particles known as atoms. Atoms from one element share similar mass, size and other properties; compound formation involves interactions among these atoms in whole number ratios.
Chemical reactions involve rearrangements of atoms; no new ones form during chemical reactions and no atoms are lost either during their creation or destruction. Dalton’s theory provided an excellent basis for understanding chemical reactions as well as material behavior, without providing insight into their internal structures.
As technology and scientific knowledge advanced, it became evident that atoms weren’t as unidirectional as previously imagined. By the nineteenth and 20th centuries breakthrough experiments demonstrated subatomic particles within them.
Electrons In 1897, J.J. Thomson first identified electrons by using cathode ray tubes. He noted the negatively charged particles orbited around atoms’ nuclei.
Ernest Rutherford first discovered Protons in 1911 with his iconic Gold Foil Test, which revealed that all atoms possess dense positively charged nuclei within them containing protons that carry positive charges.
James Chadwick discovered neutrons, electrically neutral particles located inside an atomic nucleus, during 1932.
Niels Bohr (1885 -1977), was an influential Danish scientist who first proposed his concept of an atom in 1913 based on studies into particles such as electrons and nuclei. Bohr’s Model, commonly referred to as “Bohr model”, proposed the theory that electrons have various energy levels or shells within which their energies reside – an idea known by his colleagues at Copenhagen as “The Bohr Model.” Based on this model:
Electrons orbit around nuclei with distinct energies or shells around them; those located closer to the nucleus have lower energies while electrons in outer shells possess greater ones.
Electrons absorb and emit energy in discrete bundles known as quanta, as they move between energy levels.
Also Read: the-atom-a-fundamental-building-block-of-matter
Bohr’s model could explain spectral lines found in hydrogen gas that had long remained an open question in physics; however, its limitations prevented it from fully explaining how complex molecules behaved.
Quantum Mechanical Model
Wave-Particle Duality
In the early 20th century, quantum mechanics heralded a revolutionary change to physical theory. Scientists such as Max Planck and Albert Einstein proposed the theory that electromagnetic radiation containing light had both particles and wavelike characteristics; this distinction allowed for new insights into understanding particle behavior at subatomic and atomic levels.
Schrodinger Wave Equation
Erwin Schrodinger was an Austrian scientist who invented his famous wave equation of quantum mechanics around 1926. This equation describes electron behavior within an atom as wave functions representing probability distributions mathematically – providing one of the cornerstones for quantum theory.
Electrons in an atom do not orbit around it in any one specific path; rather they reside within space-time regions determined by probabilities distribution. A wave function provides details regarding an electron’s location, spin rate and energy source.
Quantum numbers (n, represented by letters l, M and s) refer to the different quantum states and properties associated with electrons within an atom.
Schrodinger model of quantum mechanical theory offers more accurate and complete descriptions of atomic structure and behavior compared to Bohr model.
Understanding Atoms
In order to fully appreciate what makes up an atom, it’s necessary to explore both their properties and structural components.
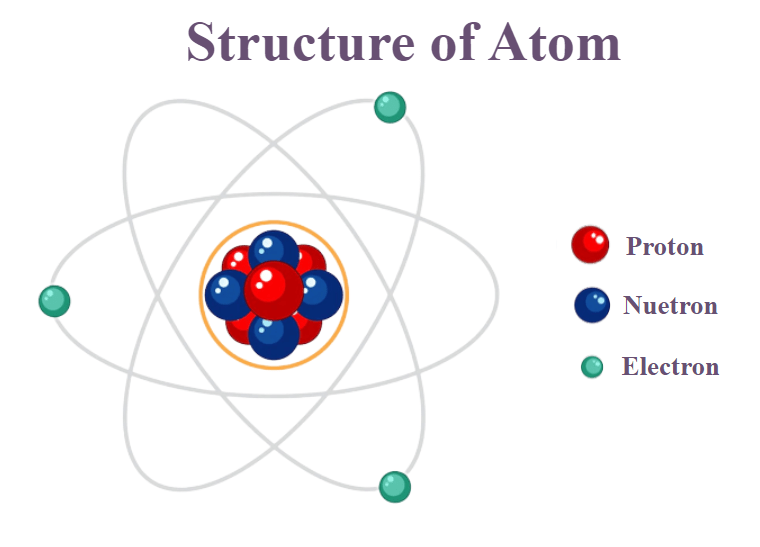
Nucleus At the core of every atom lies its nucleus: an extremely compact core composed of neutrons and protons that holds most of an atom’s mass yet occupies only a minor percentage of its space. Protons possess positive electrical charges while neutrons remain electro-neutral – these charged particles compose its nucleus while neutrons remain electro-neutral. The mass contained by its nucleus contributes significantly towards mass as well as the size of an atom’s overall structure.
Electrons
Subatomic particles with negative charges known as electrons orbit around an atom’s nucleus at specific energy levels known as electron shells. Each shell subdivides further to house specific number of electrons; their configuration determines both chemical properties and reactivity for any given atom.
Atomic Quantity
An element’s Atomic Number can be understood to represent its number of proton particles present within its nucleus and uniquely differentiate it in the periodic table. As its atomic numbers increase so does their proton count increase as well.
Isotopes
Isotopes of an element are defined as atoms with equal numbers of protons but differing amounts of neutrons; this results in different mass atomics which in turn display slightly differing chemical properties due to differences between their mass atoms.
The Periodic Table: Organising Atoms
One of the best tools available to understand and predict how atoms behave is found within the periodic table.
Dmitri Menedeleev Contribution
Russian Chemist Dmitri Mendeleev is widely acknowledged to have created the periodic table during the early 19th century. By organizing elements according to mass atomic, and noting when similar properties occurred frequently among them he could determine properties yet unknown and demonstrate regularities within chemical characteristics that had yet to be discovered.
Also Read: microsoft-onedrive-a-comprehensive-guide-to-cloud-storage-and-collaboration
Modern Periodic Table
The modern periodic table is organized based on its atomic number – this measures how many protons exist within an atom’s nucleus – as measured by its atomic number (measurement). Each period and group are further subdivided, grouping together elements with similar characteristics in columns or periods respectively. Furthermore, each detail such as Atomic symbol, Atomic number, Mass and Electron configuration for every element are displayed clearly within this chart.
Chemical Bonding and Molecular Structure
Atoms do not typically exist alone – instead they combine with one another to form molecules, making up matter itself. Understanding how atoms connect is vital in order to fully grasp its essence.
Covalent Bonds Covalent bonding occurs between molecules when electrons exchanged are stable enough to form an electron configuration that remains secure over time; it’s most often observed among nonmetals-based compounds.
Ionic Bonding Ionic bonding occurs when an atom exchanges electrons with another, creating charged Ions which attract one another because of their opposite charges, creating compounds of Ionic bonding that contain Ionics.
Metallic Bonding Metallic bonding is characteristic of metals. This process entails delocalized electrons moving freely around negatively charged metalions. Metals offer various unique properties like malleability and conductivity that make this form of connection particularly advantageous.
Molecular Geometry Molecule’s internal arrangement – also referred to as molecular geometry – plays an integral part of their chemical properties, with aspects like molecular geometry having an enormous influence. Electron pairs surrounding an atom determine its molecular geometry – linear, trigonal planar tetrahedral are amongst many others that each have distinct shapes and bond angles that define its presence within molecules.
Intermolecular Forces
Beyond chemical bonds between molecules there exist intermolecular forces which keep liquid and solid states together by keeping molecules close enough together to interact, these include forces such as:
Van der Waals Forces result from fluctuations in electron distribution which produce temporary negative and positive charges on molecules and atoms, creating Van der Waals forces which include London dispersion forces as well as dipole-dipole interactions.
Hydrogen Bonds Can Be Defined As Dipole-Dipole Interactions When hydrogen becomes attached to electron-negative elements like nitrogen, oxygen or fluorine in molecules and attracts another electronegative atom that exists elsewhere within another molecular, dipole-dipole interactions are established and hydrogen bonds become established.
Polarity and Solubility Electron distribution within molecules can result in variations of electronegativity that result in nonpolar or polar molecules, respectively. Polar molecules display unbalanced distributions of charge while nonpolar ones exhibit more uniform distribution. This characteristic determines solubility across solvent types as well as interactions with nonpolar or polar molecules.
Chemical Reactions and Atoms
Chemical reactions involve breaking and forming chemical bonds to transform raw reaction substances into end products. Atoms’ and molecules’ behavior during chemical reactions is guided by key principles like mass conservation and precise proportionality law.
Conservation of Mass According to Conservation of Mass, chemical reactions produce no net change in mass over time, as no new atoms are formed during chemical reactions; rather they rearrange to form new substances and change composition over time.
Stoichiometry
Stoichiometry is the study of quantitative relationships between reactants and products during chemical reactions. Chemists employ moles as units of measure when studying this subject in order to weigh chemical equations accurately as well as determine amounts involved.
Chemical Equations
Reactants and products in chemical reactions can often be described by using chemical equations, providing a concise way of outlining both products, reactants and the stoichiometry involved with reactions. Chemical equations typically come equipped with coefficients which show exactly which components make up each side of an equation – this ensures there’s always one answer when solving chemical equations!
Types of Chemical Reactions Chemical reactions can be divided into various groups. Some examples are:
Combine Reactions When two or more substances react, they form one product.
Descomposition Reactions A discomposition reaction occurs when one chemical compound is broken apart into multiple, simpler substances.
Single displacement reactions involve one element replacing another within an encapsulation system.
Double displacement reactions involve two distinct compounds exchanging their ions to produce two new substances.
Redox Reactions: Redox (reduction-oxidation) reactions involve the transfer of electrons among reactants to change their oxidation states and bring about changes.
Atomic Spectroscopy: Analyzing Atoms
Atomic spectroscopy is an efficient means for scientists to study how energy is distributed within an atom by studying their radiation output or absorption properties.
Emission Spectroscopy
Emission spectroscopy is the study of light emission from excited molecules. When exposed to extreme conditions of energy such as discharge of electricity or heating, their electrons become excited, jumping to higher energy states before returning back down towards normal by emitting electromagnetic radiation in either ultraviolet or visible wavelengths – which allows scientists to locate elements and calculate how many are present within a sample sample by studying this spectrum of radiation emissions.
Absorption Spectroscopy
Absorption spectroscopy investigates how certain wavelengths of light absorb specific atoms as their energies shift between higher and lower energy states, giving scientists insight into identifying various elements and measuring their amounts within samples. By measuring how much of each type of light wavelength was absorbed during this transition period, researchers can gain an insight into which elements might exist as well as determine their presence and measure them within samples.
Atomic Spectroscopy Atomic spectroscopy offers numerous applications in science and medicine. From analysing environmental conditions and material testing, to forensic investigation and pharmaceutical research. Atomic spectroscopy allows precise yet nondestructive element analyses making it an indispensable instrument in modern analytical chemistry.
Atoms in Modern Science and Technology
Atoms play an indispensable part in understanding our physical universe, while being implemented into various fields of science, engineering, technology and other disciplines.
Nanotechnology entails manipulating materials at the nanoscale level using molecules and atoms as building blocks; this creates novel materials with novel characteristics like quantum dots and carbon nanotubes with applications across electronics, medicine and materials science fields.
Nuclear Energy
Nuclear energy is an innovative method for harnessing the immense amounts of energy generated from nuclear reactions involving either fission (breakage of nuclei in an atomic chain) or fusion (combination), both which produce immense quantities of power. While fission reactions are used primarily in power plants for electricity generation purposes, scientists continue research fusion energy as potential green and sustainable power source.
Understanding atoms and molecules is fundamental to comprehending materials science research, where researchers create new materials with properties tailored to suit specific applications such as construction, aerospace electronics devices or electronic waste disposal systems. Such studies form part of this field’s foundation.
Semiconductor Devices
Semiconductor devices – like microchips and transistors – depend on the precise movement of electrons within semiconductor materials like silicon for their operation, providing the foundation of electronic technology as we know it today and revolutionizing computing, communications and information technologies in general.
Future Directions in Atomic Research
Knowledge about atoms continues to expand as research expands our understanding. Some current areas of interest in research on atomic physics include:
Quantum Computing
Quantum computing focuses on exploiting the quantum properties of quantum bits or qubits which, thanks to superposition principles and Entanglement, allow multiple states to exist at once. When coupled with conventional computers, quantum computers can help solve difficult problems more rapidly than is ever possible before.
Fundamental Particle Research
Particle physics research at facilities like the Large Hadron Collider (LHC) seeks to uncover basic components and forces influencing matter’s composition while offering insight into its origin as well as into possible explanations for dark matter or energy sources. Such experiments shed light on both.
Researchers continue to create groundbreaking materials with impressive properties, like superconductors which conduct electricity without resistance, or metamaterials which enable sound or light modulation through innovative applications.
Conclusion
Once considered an abstract and indestructible concept, atoms have now become an essential element in science and technology today. Their understanding has evolved from ancient philosophical perspectives into an accurate quantum mechanical model that depicts their actions more precisely. Atoms form the basic building blocks of matter; their interactions influence physical and chemical reactions that impact all our daily lives.
As humans endeavor to explore the subatomic world and apply our understanding to areas like nanotechnology, nuclear energy and quantum computation – discovering its mysteries remains fascinating and discoveries made every day are testaments of scientific curiosity that allow us to unlock them further and harness its potential to enhance life on Earth and deepen understanding of the universe.